The correct answer is C, This information tells us that carbon-14 is an isotope of carbon-12. Isotopes are atoms with the same atomic number but different mass number. Atomic number is the number of protons in the nucleus of an atom. Mass number is the total number of protons and neutrons in an atomic nucleus. Both carbon-14 and carbon-12 have 6 protons, 6 electrons, but they differ in the fact that carbon-14 has 8 neutrons as opposed to 6 neutrons in carbon-12.
mass number of
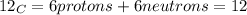
mass number of
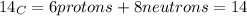 .
.