To determine the moles of NaOH we must review the stoichiometry of the reaction.
In the first place we must verify that the reaction they give us is balanced, for this we count the atoms of each element on each side of the reaction.
We have to.
Reagents:
K - 1 atom
H - 6 atoms
C - 8 atoms
Na - 1 atom
Products:
K - 1 atom
H - 6 atoms
C - 8 atoms
Na - 1 atom
Indeed, the reaction is balanced. Now we can continue.
We see that for each mole of KHP that reacts, one mole of NaOH is needed, that is, the ratio is 1 to 1.
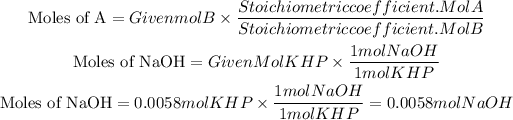
Therefore, if 0.0058 moles of KHP react, the necessary moles of NaOH will also be 0.0058 mol.
Moles of NaOH=0.0058mol