Answer: A. positive enthalpy change and positive entropy change
Step-by-step explanation:
According to Gibb's equation:
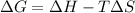
 = Gibbs free energy
= Gibbs free energy
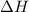 = enthalpy change
= enthalpy change
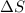 = entropy change
= entropy change
T = temperature in Kelvin
 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 = -ve, reaction is spontaneous
= -ve, reaction is spontaneous
 = 0, reaction is in equilibrium
= 0, reaction is in equilibrium
Thus when
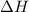 = +ve
= +ve
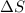 = +ve
= +ve
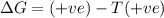
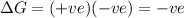 only when
only when
 is higher which is possible at high temperatures
is higher which is possible at high temperatures
Reaction is spontaneous at high temperatures when positive enthalpy change and positive entropy change is there.