Answer:
53.97 moles
Step-by-step explanation:
First off, it is important to understand the following concepts;
Avogadro's number is a proportion that relates molar mass on an atomic scale to physical mass on a human scale.
Avogadro's number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. It is equal to
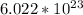 mol-1 and is expressed as the symbol NA.
mol-1 and is expressed as the symbol NA.
Hence, the relationship between number of moles and number of atoms is given as;
1 mole =
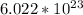 atoms
atoms
The question asks to find the number of moles
 atoms equals to.
atoms equals to.
so we have;
1 =
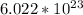
x =

upon cross multiplication, we are left with;
x = (1 *
 ) /
) /
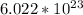
x = 53.97 moles