Answer:
The correct answer is option C.
Explanation:
To calculate density of a substance, we use the equation:
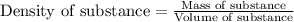
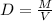
We are given:
Density of liquid = D
Mass of copper= 89.6 g
Volume of copper =
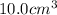
Putting values in above equation, we get:
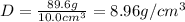
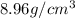 is the density of copper.
is the density of copper.