Answer: The mass of butane is more than the mass of propane and both the compounds have same number of molecules.
Step-by-step explanation:
We are given:
Moles of propane = 5 moles
Moles of butane = 5 moles
According to mole concept:
1 mole of a compound contains
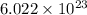 number of molecules
number of molecules
So, 5 moles of propane will contain =
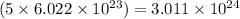 number of molecules.
number of molecules.
And, 5 moles of butane will contain =
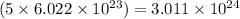 number of molecules.
number of molecules.
To calculate the mass for given number of moles, we use the equation:
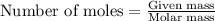 .....(1)
.....(1)
Moles of propane = 5 moles
Molar mass of propane = 44 g/mol
Putting values in equation 1, we get:
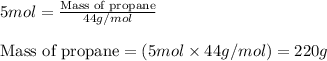
Moles of butane = 5 moles
Molar mass of butane = 58 g/mol
Putting values in equation 1, we get:
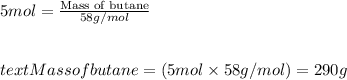
Mass of butane > Mass of propane
Hence, the mass of butane is more than the mass of propane and both the compounds have same number of molecules.