Answer: The correct answer is CO.
Step-by-step explanation:
Incomplete combustion is defined as the type of reaction in which a hydrocarbon reacts with oxygen to produce carbon monoxide, water and carbon. In this type of combustion, insufficient amount of oxygen is provided to the hydrocarbon.
General equation for incomplete combustion reaction follows:
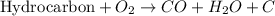
Water as a product is obtained in complete combustion as well. So, if in a chemical reaction, carbon monoxide or carbon are produced, it will result in incomplete combustion.
Thus, the correct answer is CO.