Answer:
Option D is correct. Magnesium Chloride
Step-by-step explanation:
A substance that produces an electrically conducting solution when dissolved in a polar solvent for instance, water is known as an electrolyte.
During the electrolytic process, ionic compounds decompose into their elemental form when charged with an electric current.
For instance, Magnesium Chloride dissociate into its molten state and this molten ionic compound separates into elements through the oxidation and reduction process as shown by the chemical reaction below;
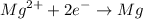
Magnesium gains two electrons from the reaction to form the magnesium atom. Similarly, Chlorine ion oxidizes by losing electrons to form chlorine gas as shown:
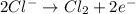
According to the question, we can see that the electrolytes contain positive ions of +2 floating and negative ions of -1. Since the charge of Magnesium ion is +2 and the charge of chloride ion is -1, hence we can conclude that the electrolytes that would represent this image is Magnesium Chloride