Answer : The correct option is, (D) 176 grams
Solution : Given,
Mass of Fe = 112 g
Mass of S = 64 g
Molar mass of Fe = 56 g/mole
Molar mass of S = 32 g/mole
Molar mass of FeS = 88 g/mole
According to the Law of conservation of matter, in the chemical reaction, the total mass of the products must be equal to the mass of the reactants.
The given balanced chemical reaction is,
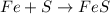
From the reaction, we conclude that the 1 mole of iron, (Fe) react with the 1 mole of sulfur, (S) to give 1 mole of iron sulfide, (FeS).
First we have to calculate the moles of Fe and S.
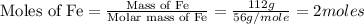
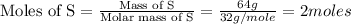
The moles of Fe and S are same. From the reaction, we conclude that
As, 1 mole of Fe react to give 1 mole of FeS
So, 2 moles of Fe react to give 2 moles of FeS
Now we have calculate the mass of FeS.
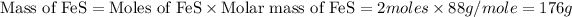
Therefore, the number of grams of iron sulfide (FeS) produced in this reaction will be, 176 grams.