Answer: Option (a) is the correct answer.
Step-by-step explanation:
A reaction where heat energy is absorbed by the molecules of a substance is known as an endothermic reaction.
The value of
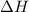 is positive for an endothermic process. This also means that a decrease in temperature occurs with absorption of heat.
is positive for an endothermic process. This also means that a decrease in temperature occurs with absorption of heat.
Whereas when energy is released by the molecules of a substance in a reaction then it is known as an exothermic reaction.
The value of
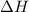 is negative for an exothermic process. This means an increase in temperature occurs with release of heat energy.
is negative for an exothermic process. This means an increase in temperature occurs with release of heat energy.
Thus, we can conclude that temperature increases indicates that an exothermic reaction has occurred.