Answer:
The answer is 1,5 moles of H2
Step-by-step explanation:
Let's start from the definition of the Avogadro's number. The Avogadro's number is 6,02 x
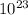 and means that, for any compound, we have 6,02 x
and means that, for any compound, we have 6,02 x
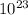 molecules for each mol. So, in that way, we can write the following expression:
molecules for each mol. So, in that way, we can write the following expression:
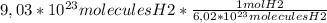
The result is: 1,5 moles of H2