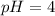Answer :
(A) The number of moles of
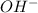 ions per liter is, 0.1 moles/L
ions per liter is, 0.1 moles/L
(B) The number of molecules of
 ion is,
ion is,
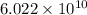
(C) The pH of the solution will be, 4
Solution for part A :
First we have to calculate the pOH of the solution.
As we know that,
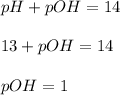
Now we have to calculate the moles of
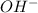 ion per liter.
ion per liter.
![pOH=-\log [OH^-]\\\\1=-\log [OH^-]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/y1112trh4pnfiv8ov4fdgi9sh1mxpta3nm.png)
![[OH^-]=0.1moles/L](https://img.qammunity.org/2018/formulas/chemistry/middle-school/qcuha3ic02gdnlvanudww7nfs9gog2qwtm.png)
Solution for part B :
First we have to calculate the
 ion concentration.
ion concentration.
![pH=-\log [H^+]\\\\13=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/araulae5ai7yl4gg4zmia9ojiz90vmnykj.png)
![[H^+]=10^(-13)moles/L](https://img.qammunity.org/2018/formulas/chemistry/middle-school/qhhk970y5c61lhwgcdb3fseh25kgsmz8ux.png)
Now we have to calculate the number of molecules of
 ion
ion
As, 1 mole contains
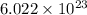 number of molecules of
number of molecules of
 ion
ion
So,
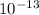 moles contains
moles contains
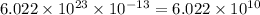 number of molecules of
number of molecules of
 ion
ion
Solution for part C :
![pH=-\log [H^+]\\\\pH=-\log (1* 10^(-4))](https://img.qammunity.org/2018/formulas/chemistry/middle-school/h6r2yc10lo8xi21okht794powc3luxnqh2.png)