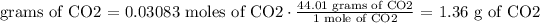1) Balance the equation.
NaHCO3 + HC2H3O2 => Na+ + C2H3O2 + H2O + CO2
2) Find out the molar mass of NaHCO3
Na = 22.990 g/mol
H = 1.00794 g/mol
C = 12.0107 g/mol
O = 15.9994 g/mol
The molar mass of NaHCO3 is
(22.990) + 1.00974 + 12.0107 + (15.9994 * 3) = 84.0066 g/mol
3) Convert 2.59 g of NaHCO3 into moles
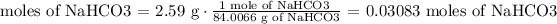
4) Use use the stoichiometric relationship between NaHCO3 and CO2 to find out the potential production of CO2
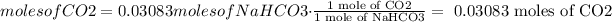
5) Convert moles of CO2 into grams of CO2. We need the CO2 molar mass.
The molar mass of CO2 is = 44.0095 g/mole