We are required to calculate the molarity (or concentration).
Given:
mass = 12.90 g
volume = 2130.0 mL
molar mass = 189.7 g/mol
The formula for molarity:
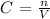
where n is the number of moles and v is the volume.
We can calculate the number of moles:
n = m/M where m is the mass and M is the molar mass
n = 12.90g/189.7g/mol
n = 0.0680 mol
To calculate the molarity we need to convert the volume from mL to L
v = 2130.0 mL = 2.130 L
C = n/V
C = 0.0680mol/2.130L
C = 0.0319 mol/L (or M)
Therefore the molarity = 0.0319 mol/L