Answer: A) As the marshmallow was heated the particles moved faster increasing its volume.
Step-by-step explanation: When heat is supplied to the marshmallows, the kinetic energy of the marshmallows increase and thus they start moving randomly and thus the molecules occupy more volume and volume increases.
As we know kinetic energy is directly related to temperature, thus as temperature is increased, the kinetic energy of the molecules also increase.
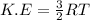
R = gas constant
T= temperature