Answer
77.8 grams
Explanation
The given balanced chemical equation is:
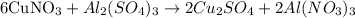
From the balanced chemical equation for the reaction, 6 moles of copper (I) nitrate (CuNO₃) produced 2 moles of aluminum nitrate (Al(NO₃)₃).
That is, mole ratio of CuNO₃ to Al(NO₃)₃ is 6 : 2
Note that:
Molar mass of CuNO₃ = 125.55 g/mol
Molar mass of Al(NO₃)₃ = 212.996 g/mol
For CuNO₃
It implies that 1 mole of CuNO₃ = 125.55 g
Therefore, 6 moles of CuNO₃ will be = 6 x 125.55 g = 753.30 g
Also for Al(NO₃)₃
It implies that 1 mole of Al(NO₃)₃ = 212.996 g
So 2 moles of Al(NO₃)₃ = 2 x 212.996 g = 425.992 g
Now, we shall calculate the number of grams of copper (I) nitrate (CuNO₃) required to produce 44.0 grams of aluminum nitrate (Al(NO₃)₃) as follows:
Let the number of copper (I) nitrate (CuNO₃) required to be x
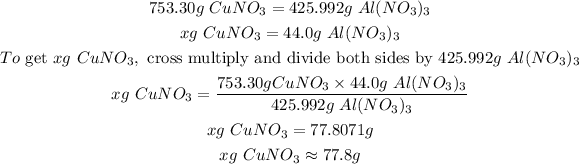
Hence, 77.8 grams of copper (I) nitrate (CuNO₃) are required to produce 44.0 grams of aluminum nitrate (Al(NO₃)₃).