Answer:
n = 2.11 moles
Step-by-step explanation:
As we know by the reaction of hydrogen and nitrogen
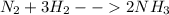
so here from above equation we know that for complete conversion of 3 moles of hydrogen we need 1 mole of nitrogen
so here we know that 6.34 moles of hydrogen is available
so we require 1/3 times of moles of nitrogen for complete reaction
so number of moles of hydrogen will be
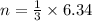
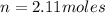
so here we can say it requires 2.11 moles of nitrogen for complete reaction of 6.34 moles of hydrogen