Answer: 0.3794 moles of Iodine gas are produced.
Step-by-step explanation:
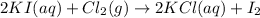
Volume of iodine gas produced at STP =8.5 L
At STP, the 1 mol of gas occupies volume = 22.4 L
So, 8.5 L of volume will be occupied by:
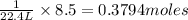
0.3794 moles of Iodine gas are produced.