Answer : The correct option is, (D)
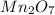
Explanation :
Binary ionic compound : It is a type of compound that is made up of the ions of the two different elements in which one element is a metal and another element is a non-metal. It is only made up of two elements.
From the given options, option (A)
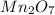 is a binary ionic compound because in this compound Mn is a metal which combine with the an oxygen non-metal element.
is a binary ionic compound because in this compound Mn is a metal which combine with the an oxygen non-metal element.
While the other options are not a binary compound because it is made up of more than two elements.
Hence, the
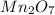 is a binary ionic compound.
is a binary ionic compound.