Answer: The correct answer is Option 1.
Step-by-step explanation:
We are given two intermediate equations and a final net equation for the formation of calcium carbonate. To form a net equation, we have to apply some operations on the second equation.
Two intermediate equations given are:
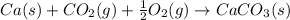
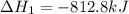
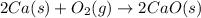
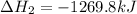
To get the final equation, we will half the second equation and reverse the equation, we get:
Equation 1:
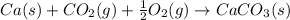
Equation 2:
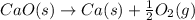
Net Equation:
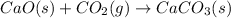
Hence, the correct answer is Option 1.