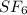Answer:The molecular formula of sulfur hexafluoride is
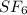
Step-by-step explanation:
Electronic configuration of sulfur is gives as:
![[S]=1s^22s^2p^63s^23p^4](https://img.qammunity.org/2018/formulas/chemistry/high-school/d490l0udhf8qjedtogjqtqpzjbjap5d9wp.png)
Sulfur has 6 valence electrons. It can either donate its 6 electrons or accept 2 electrons to gain fully filled electronic configuration stability.
In Sulfur hexafluoride, there are 6 fluorine atoms and 1 sulfur atom.Fluorine being most electronegative atom will attract the valence electrons of sulfur towards itself.
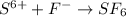
The molecular formula of sulfur hexafluoride is