ANSWER
The concentration of the solution is 0.042M
Step-by-step explanation
Given that;
The volume of the solution is 600mL
The mass of the solute is 2.50 g
To find the concentration of the solution, follow the steps below
Step 1; Find the number of moles of CaCO3 using the below formula
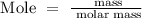
Recall, that the molar mass of CaCO3 is 100.0869 g/mol
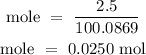
The number of moles of the solute is 0.0250 mol
Step 2; Convert the volume of the solution to L from mL
Recall, that 1mL is equivalent to 0.001L
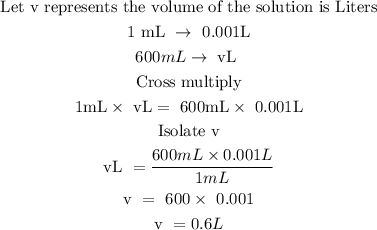
The volume of the solution in L is 0.6L
Step 3; Find the concentration of the solution using the below formula
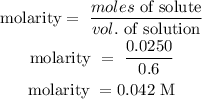
The concentration of the solution is 0.042M