Answer: The atom will have a full valence electron shell.
Step-by-step explanation:
Covalent bond is defined as the bond which is formed after the sharing of electrons takes place between the atoms. This is usually formed between two non-metals.
For Example: HCl is formed by the combination of hydrogen and chlorine atoms.
Hydrogen is the 1st element of the periodic table having electronic configuration of
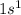
Chlorine is the 17th element of the periodic table having electronic configuration of
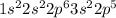
Both the elements require 1 electron to complete their valence shell. Thus, both the elements will share electrons in order to form a stable compound of hydrogen chloride.
Hence, the atom will have a full valence electron shell.