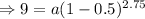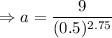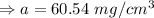Answer:
The density of the substance when it was deposited 55 years ago was 60.54 mg/cm³
Explanation:
The exponential function for growth and decay is,
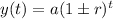
where,
y(t) = the amount after time t
a = initial amount
r = rate of change
t = time period
+ is used for growth and - is used for decay.
As this is the case of decay, so the function becomes,
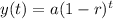
Given,
y(55) = 9 mg/cm³
r = 50% = 0.5 (as the substance is getting halved)
t =
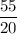 = 2.75 (as the half life is 20 years and we have convert time in terms of half life)
= 2.75 (as the half life is 20 years and we have convert time in terms of half life)
Putting the values,