First, we have to remember that the pH is a scale that allows us to determine if a solution is acid or basic.
The equation for calculating the pH is:
![pH=\text{ -log \lparen\lbrack H}^+])\text{ }](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/l92j42ld2nc2lsga9l6n.png)
Being [H+], the concentration of hydronium ion.
Then, we have all the information to calculate the pH of the solution:
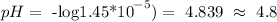
Then, the answer is that the vinegar solution's pH is equal to c. 4.8