Answer:
See explanation.
Step-by-step explanation:
Hello,
In this case, after looking for the information, the four cases and their discussion are:
a. Potassium carbonate and hydrochloric acid : in this case, the chemical reaction is:
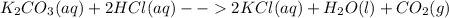
Aqueous potassium chloride and water are formed and carbon dioxide is given off as gas due to the instability of the carbonic acid.
b. Zinc chloride and silver nitrate : in this case, the chemical reaction is:

Aqueous silver nitrate is formed and solid silver chloride is precipitated out due to its low solubility in water.
c. Magnesium chloride and sodium hydroxide : in this case, the chemical reaction is:
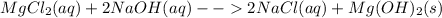
Aqueous sodium chloride is formed and solid magnesium hydroxide is precipitated out due to its low solubility in water.
d. Ammonium nitrate and sodium hydroxide: in this case, the chemical reaction is:
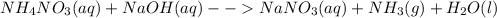
Sodium nitrate becomes aqueous as liquid water is formed and ammonia is given off as a gas due to the instability of the ammonium hydroxide.
Best regards.