Answer: Option (C) is the correct answer.
Step-by-step explanation:
When particles move from their initial position then the energy held by them is known as kinetic energy.
Kinetic energy of molecules of a substance is directly proportional to temperature.
Mathematically, K.E =
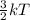
where k = boltzmann constant
T = temperature
Therefore, an increase in temperature will increase the kinetic energy of particles.
Therefore, we can conclude that the statement kinetic energy decreases as temperature decreases, correctly describes what is happening in the given system.