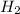Answer: The correct answer is 1.32 mol
Step-by-step explanation:
For the reaction of lithium and hydrofluoric acid, the equation follows:
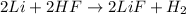
By Stoichiometry of the reaction:
if 2 moles of lithium is producing 1 mole of hydrogen gas,
Then, 3.50 moles of lithium will produce =
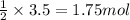 of hydrogen gas.
of hydrogen gas.
- Now, to know the theoretical yield of hydrogen gas, we use the equation:
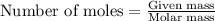 ...(1)
...(1)
Moles of hydrogen gas = 1.75 mol
Molar mass of hydrogen gas = 2 g/mol
Putting values in above equation, we get:

- To calculate the percentage yield, we use the equation:
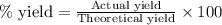
Percentage yield = 75.5 %
Theoretical yield = 3.5 g
Putting values in above equation, we get:

- Now, calculating the moles of hydrogen gas, we put the value in equation 1, we get:
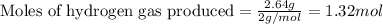
Hence, the correct answer is 1.32 mol