To solve this question, like the previous one, we will use the ideal gas law. In this case we are given the moles (n) and volume(V) of the initial state and asked to find the moles of the final state.
We will assume that there is no change in temperature and since the balloon is flexible as the volume increases, the pressure will remain constant, therefore we have.
Initial state:
n1=10 moles
V= 18L
Final state:
n2=20 moles
V=?
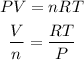
The RT/P ratio will be constant in both initial and final states, therefore we can equate the states as follows:
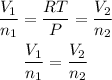
We clear V2 and replace known data:
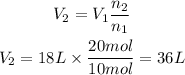
The new volume in liters is 36L