Step-by-step explanation:
The collection of elements on the right side of periodic table is known as p-block elements.
General electronic configuration of p-block elements is
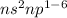 . And, these elements are from group 13 to group 18.
. And, these elements are from group 13 to group 18.
For example, nitrogen has atomic number 7 and it is a group 15 element. Electronic configuration of nitrogen is
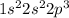 .
.
Since, nitrogen lies in between group 13 to 18. So, it is a p-block element.
Thus, we can conclude that the name of the collection of elements on the right side of the periodic table with electrons sequentially filling orbitals in their valence is p-block elements.