Answer:
At pH=7, number of hydrogen ions would be equal to number of hydroxide ions in a solution.
Step-by-step explanation:
We know that at 25
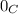 ,
,
![[H^(+)][OH^(-)]=10^(-14)](https://img.qammunity.org/2018/formulas/chemistry/middle-school/rt8k4i1qq2ewyc5drfhr34wfvfnnodisf5.png)
where species inside third bracket represent concentrations in molarity
When number of hydrogen ion will be equal to number of hydroxide ions then
![[H^(+)]=[OH^(-)]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/hblvsqthpicv0nvhag0otm5dsti6baw657.png)
So
![[H^(+)]^(2)=10^(-14)](https://img.qammunity.org/2018/formulas/chemistry/middle-school/neuk4qjbbh5p3q1pi361vnturad5wrkz3o.png) or
or
![[H^(+)]=10^(-7)M](https://img.qammunity.org/2018/formulas/chemistry/middle-school/1upkejsyrg6mmz48ns30lejsimugxd7kjv.png)
Hence pH=
![-log[H^(+)]=-log[10^(-7)]=7](https://img.qammunity.org/2018/formulas/chemistry/middle-school/vqzqky84n5rosefts73iyec9v6tyo7vj5z.png)