Step-by-step explanation:
Ionic bonds are defined as the bonds which are formed by the complete transfer of electrons from cation (positively charged ions) to anion (negatively charged ions).
This bond is formed by the attraction of oppositely charged species. They are usually formed between a metal and a non-metal.
For Example: NaCl,
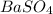 etc...
etc...
Covalent bond is defined as the bond which is formed by the sharing of electrons between the atoms.
They are usually formed between two non-metals.
For Example: HCl,
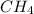 etc.
etc.