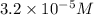Answer : The hydrogen ion concentration is, (B)
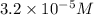
Explanation :
pH : It is defined as the negative logarithm of hydrogen ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/y1nlg9qxar6fauop1r05a1g4xt6dhnvirc.png)
Given:
pH = 4.5
Now put the value of pH in the above expression, we get value of hydrogen ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/y1nlg9qxar6fauop1r05a1g4xt6dhnvirc.png)
![4.5=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/college/l71ec9ov77dllgfbhzrv8t2vy7rmxvol59.png)
![[H^+]=3.2* 10^(-5)M](https://img.qammunity.org/2018/formulas/chemistry/college/rv3s6fpuw7kkqt8p1csi6qj36jcnxd0obu.png)
Thus, the hydrogen ion concentration is,