Answer: Option (C) is the correct answer.
Step-by-step explanation:
When aluminium chloride reacts with cesium, then the reaction will be as follows.
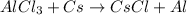
Number of reactant atoms are as follows.
Number of product atoms are as follows.
Thus, in order to balance the equation, multiply Cs by 3 on reactant side and multiply CsCl by 3 on the product side.
Therefore, the balanced chemical equation will be as follows.
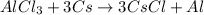
Hence, we can conclude that out of the given options, AlCl3+ 3Cs > 3CsCl + Al is the correct chemical equation.