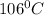Answer:
Explanation: IDEAL GAS LAW
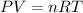
where,
P = pressure of the gas = 889 torr = 1.17 atm (1 torr=0.0013atm)
V = volume of the gas = 11.8 L
T = temperature of the gas = ? K
n = number of moles of gas = 0.444
R = Gas constant = 0.0821 Latm/moleK
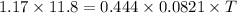
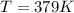

Thus temperature of gas is