Answer: The other gas is Krypton (Kr).
Explanation: The rate of effusion of gas is given by Graham's Law.
Graham's Law states that the rate of effusion of gas is inversely proportional to the square root of their atomic masses.
Mathematically,
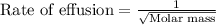
Rate of effusion is the amount of gas effused in a given time 't'
Mathematically,
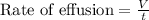
As per the question:
For Neon:
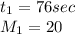
For unknown gas:
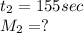
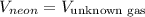
Putting values in rate of effusion formula, we get:
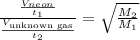
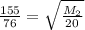
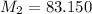
This mass corresponds to the mass of Krypton noble gas.