Answer 1:
Step-by-step explanation:
Double displacement reaction: It is a reaction in which reactants exchange their ions to form products in chemical reaction.
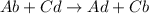
When aqueous solutions of silver nitrate and potassium chloride reacts together to form white precipitate of silver chloride with potassium nitrate in aqueous solution. While writing chemical reaction, first write the molecular formula of silver nitrate and potassium chloride with 'addition' sign in between(+) on the left hand side. Followed by right arrow and then the molecular formula of products formed on the right hand side
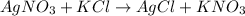
Answer 2: 1.69 moles of
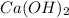 remained unreacted.
remained unreacted.
Step-by-step explanation:
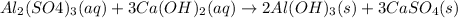
Number of moles of
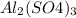 =
=
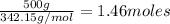
Number of moles of
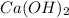 =
=
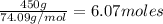
According to reaction , 1 mole of
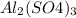 react with 3 moles of
react with 3 moles of
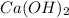 , then 1.46 moles of
, then 1.46 moles of
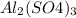 will react with :
will react with :
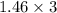 moles of
moles of
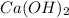 that is 4.38 moles.
that is 4.38 moles.
1.46 moles
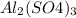 react with 4.38 moles of
react with 4.38 moles of
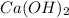 . So, this means that
. So, this means that
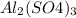 is present as limiting reagent. And
is present as limiting reagent. And
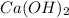 is an excessive reagent.
is an excessive reagent.
Moles of
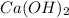 left unreacted :6.07 moles - 4.38 moles =1.69 moles
left unreacted :6.07 moles - 4.38 moles =1.69 moles
Hence,1.69 moles of
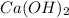 remained unreacted.
remained unreacted.