Answer: The correct option is (B).
Step-by-step explanation:
- According to Boyle's law:'Pressure of the gas is inversely proportional to the volume occupied by the gas',
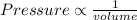
- volume is inversely proportional to the density,
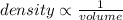
When a person squeezes and applies pressure to the outside of a balloon, air particles inside will then occupy less volume which will cause increase in the density of the particles.