Answer : The concentration of
![[H^+],[ClO_4^-]\text{ and }[OH^-]](https://img.qammunity.org/2018/formulas/chemistry/college/4h8olcwo3mdnpipil6elnkr5y3m4k6ivre.png) are,
are,
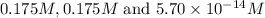 respectively.
respectively.
Solution : Given,
concentration of
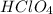 solution = 0.175 M
solution = 0.175 M
The balanced equilibrium reaction will be,
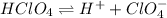
If the
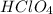 dissociates 100 percent then the concentration of
dissociates 100 percent then the concentration of
 and
and
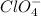 will be, 0.175 M
will be, 0.175 M
Thus,
![[H^+]=[ClO_4^-]=0.175M](https://img.qammunity.org/2018/formulas/chemistry/college/nnqsoua9s13tg7bgkc335mjxy4wadb87vk.png)
Now we have to calculate the pH.
![pH=-\log [H^+]\\\\pH=-\log (0.175)=0.756](https://img.qammunity.org/2018/formulas/chemistry/college/tda27cftnq6amdta57z7357kyh1jlt1czf.png)
Now we have to calculate the pOH.
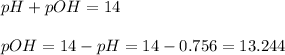
Now we have to calculate the concentration of
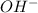 ion.
ion.
![pOH=-\log [OH^-]\\\\13.244=-\log [OH^-]](https://img.qammunity.org/2018/formulas/chemistry/college/55zp80od6phpuf9khsj00trj1lmz7g25ez.png)
![[OH^-]=5.70* 10^(-14)M](https://img.qammunity.org/2018/formulas/chemistry/college/u5ljkvmyc2obctmssb6pp5h5na1qp4ux5m.png)
Therefore, the concentration of
![[H^+],[ClO_4^-]\text{ and }[OH^-]](https://img.qammunity.org/2018/formulas/chemistry/college/4h8olcwo3mdnpipil6elnkr5y3m4k6ivre.png) are,
are,
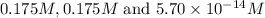 respectively.
respectively.