Answer : The correct option is, (+2)
Explanation :
The given electronic configuration is,
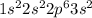
In the given electronic configuration of an atom, '3s' shell is the outermost shell in which two electrons are present. For the stable configuration, these two electrons will remove easily from the outer shell and atom will carry (+2).
Total number of electrons in the given configuration= 12 electrons
And magnesium is the element which have 12 electrons. Due to the stable electronic configuration, magnesium atom changes their electronic configuration by losing two electrons and
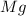 atom changes to
atom changes to
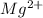 ion.
ion.
Hence, the most likely charge of an ion formed from an atom with the given electron configuration would be, (+2)