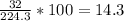Answer:
The concentration of the solution in percent by mass is 14.3%
Step-by-step explanation:
Given:
Mass of KCl (solute) = 32.3 g
Mass of water (solvent) = 192 g
To determine:
Concentration of solution in % mass i,e. %(w/w)
Formula:
%w/w
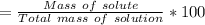
Total mass of solution = solute + solvent = 32.3 + 192 = 224.3 g
%w/w =