Answer: The correct answer is Option 3.
Step-by-step explanation:
Common ion effect is defined as the effect which occurs on equilibrium when a common ion (an ion which is already present in the solution) is added to a solution. This effect decreases the solubility of a solute.
In silver nitrate, the ions present in the solution are:
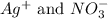
For the given options:
Option 1: 0.1 M potassium chloride
The ions formed by the ionization of this solution are
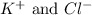 . As, no common ions are present. So, addition of this substance will not decrease the solubility of silver nitrate solution.
. As, no common ions are present. So, addition of this substance will not decrease the solubility of silver nitrate solution.
Option 2: 0.1 M barium chloride
The ions formed by the ionization of this solution are
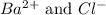 . As, no common ions are present. So, addition of this substance will not decrease the solubility of silver nitrate solution.
. As, no common ions are present. So, addition of this substance will not decrease the solubility of silver nitrate solution.
Option 3: 0.1 M sodium nitrate
The ions formed by the ionization of this solution are
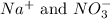 . As, nitrate ions are present which is a common ion. So, addition of this substance will decrease the solubility of silver nitrate solution.
. As, nitrate ions are present which is a common ion. So, addition of this substance will decrease the solubility of silver nitrate solution.
Option 4: 0.1 M sodium carbonate
The ions formed by the ionization of this solution are
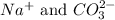 . As, no common ions are present. So, addition of this substance will not decrease the solubility of silver nitrate solution.
. As, no common ions are present. So, addition of this substance will not decrease the solubility of silver nitrate solution.
Hence, the correct answer is Option 3.