Answer: 5.6 Mev
Explanation: Binding energy is calculated by using the formula,
bonding energy = mass defect x 931 Mev
mass defect is given as 0.042 amu.
So, binding energy = 0.042 x 931 Mev = 39.012 Mev
Sum of protons and neutrons is known as number of nucleons. Li has 3 protons and 4 neutrons.
So, number of nucleons = 3+4 = 7
Binding energy per nucleon =
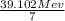
binding energy per nucleon = 5.6 Mev
So, the binding energy per nucleon for Li is 5.6 Mev where Mev stands mega electron volt.