Step-by-step explanation:
Reactants are the species that are present on left hand side of a chemical reaction. Whereas products are the species that are present on right hand side of a chemical reaction.
For example,
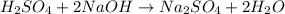
Here, both sulfuric acid and sodium hydroxide are the reactants. On the other hand, both water and sodium sulfate are the products.