Step-by-step explanation:
When a chemical bond is formed by sharing of electrons then it is known as a covalent bond.
A covalent bond is always formed between two non-metals.
For example, atomic number of chlorine is 17 and its electronic distribution is 2, 8, 7.
As a result, it needs one more electron to complete its octet. So, when it chemically combines with another chlorine atom then there will occur sharing of electrons.
Hence,
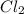 is formed which is a covalent compound.
is formed which is a covalent compound.
On the other hand, when a chemical bond is formed by transfer of electrons from one atom to another then it is known as an ionic bond. An ionic bond is always formed between a metal and a non-metal.
For example, LiCl is an ionic compound.
Thus, we can conclude that in a chlorine gas covalent bonding is present when two chlorine atoms share an electron.