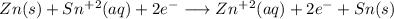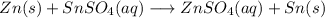Answer:
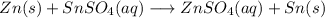
Step-by-step explanation:
Hi, when adding Zn to the solution because of its high reactivity it will displace the Sn. This process is a redox reaction.
First step is to identify the compound that oxidates and the compound that reduces. The Zn has a high oxidation potencial so it will oxidate:
Oxidation hemireaction:
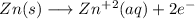
The reduction reaction will be performed by the Sn:
Reduction hemireaction:

Writting the net redox equation: