Answer:
The correct answer is the second.
Step-by-step explanation:
Oxidation occurs when an element loses electrons, acquiring a positive charge. Reduction occurs when an element gains electrons and goes from a positively charged state to a neutral one.
Aluminum metal (Al) oxidizes, so we say that Al is the reducing species, which acts by reducing
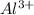 . Copper (
. Copper (
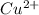 ) is reduced, so it is the oxidizing agent, it acts oxidizing to Cu.
) is reduced, so it is the oxidizing agent, it acts oxidizing to Cu.
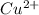 oxidizes to Cu, and Al reduces to
oxidizes to Cu, and Al reduces to
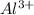 .
.
Have a nice day!