Answer:
m = 7.39 g.
Step-by-step explanation:
Hello!
In this case, since the molar mass of iron (III) phosphate is 150.82 g/mol based on its molecular formula (FePO₄), we can compute the mass in grams of 0.0490 moles of this compound by setting up the following dimensional analysis:
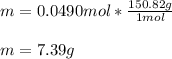
Best regards!