Option (a) is correct.
A reducing agent is the one which loses electrons to other substance and an oxidizing agent is one which accepts electrons.
Here, In
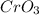
, Cr has oxidation number 6+ in the L.H.S of the equation, but on R.H.S its oxidation number is 0 i.e. it Cr has gained electrons such that total charge is 0.
And the oxidation state of Al in the left-hand side of equation is 0 and in right-hand side, it is +6.i.e. it has donated its electrons to Cr.
Hence, Cr is the oxidizing agent and Al is the reducing agent.