Answer: non polar covalent
Step-by-step explanation:
An ionic bond is formed when an element completely transfers its valence electron to another element. Example:
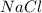
Metallic bond is the bond formed between the nucleus of metal and the free electrons. Example:
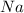
A polar covalent bond is defined as the bond which is formed when there is a difference of electronegativities between the atoms. Example:
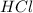
A non-polar covalent bond is defined as the bond which is formed when there is no difference of electronegativities between the atoms.Example:
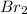
Water being polar only dissolves polar substances in it and hence bromine cannot dissolve in water.